Quadruple Whole-Cell Electrophysioloy
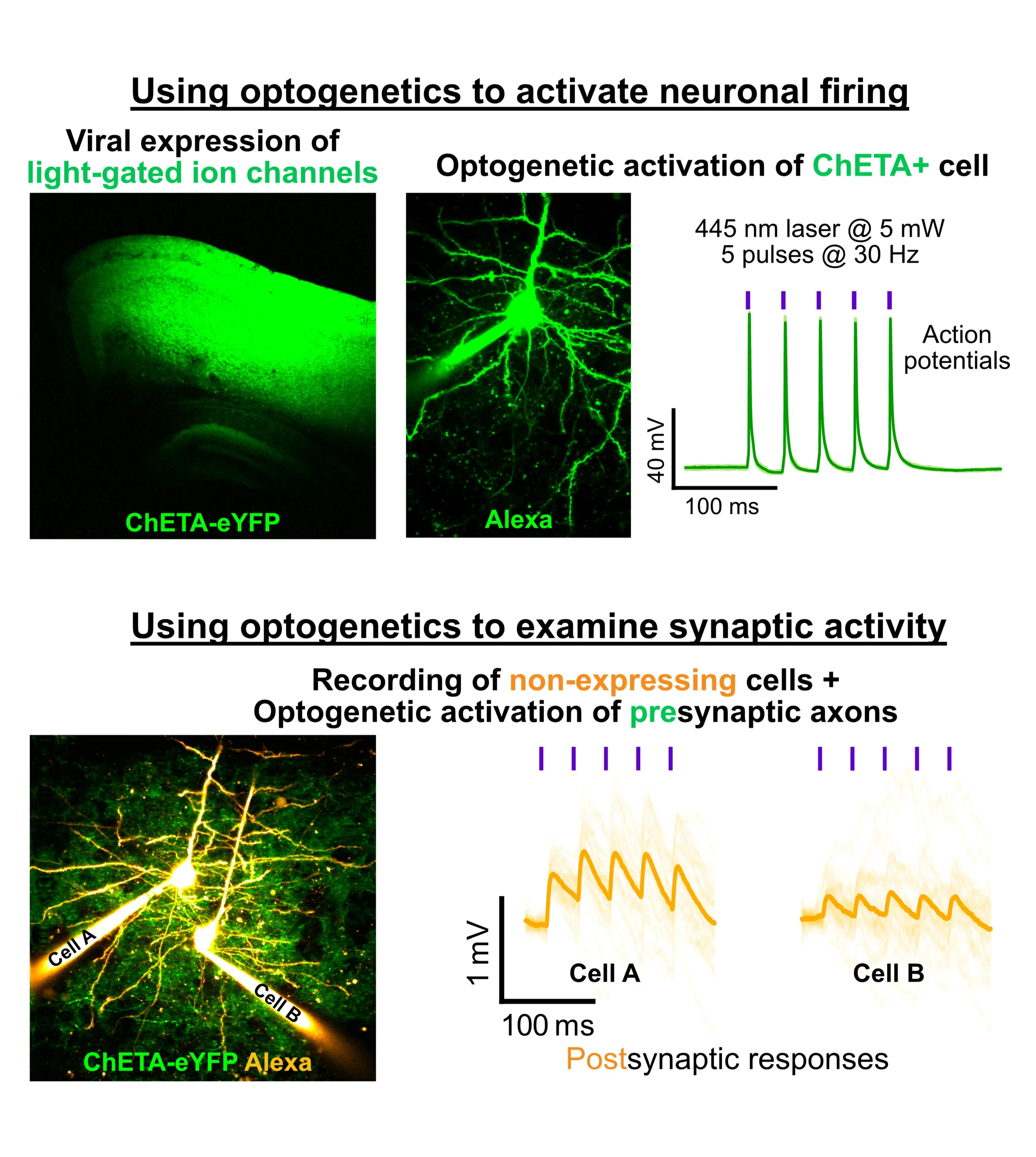
Whole-Cell Electrophysiology allows sub-millisecond and micro-volt electrical activities to be measured. These are crucial for understanding how neurons communicate via synapses and integrate inputs - neural encoding and information transfer.
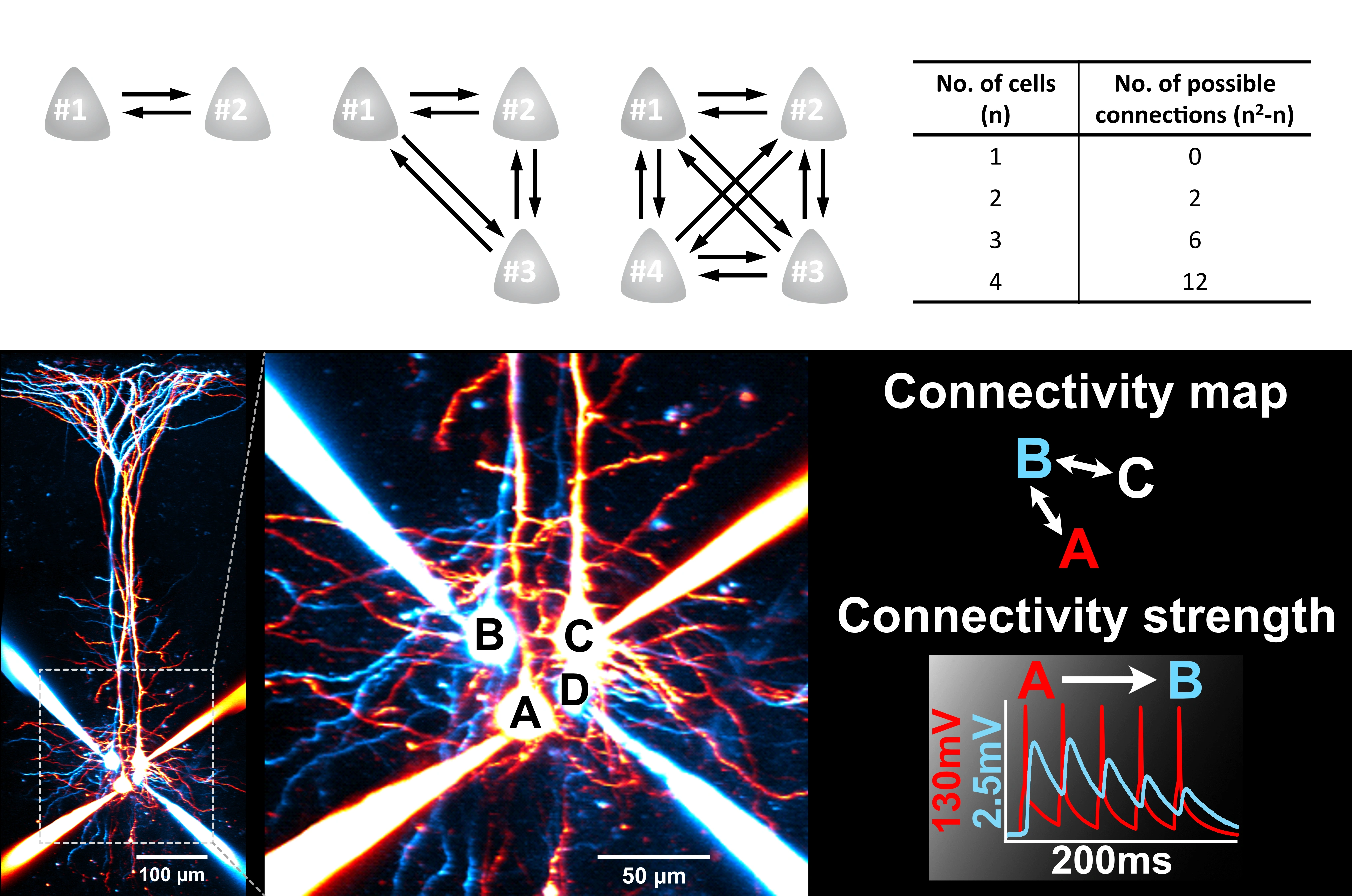
Brain connections are sparse (~10% in the neocortex). Finding connections to examine synaptic transmission is therefore difficult. Being able to patch multiple cells enables us to find connections at a much faster rate, making the impossible experiments possible.
This state-of-art technique continuous to be the gold standard for mapping circuits.