Our Research
We use an integrated approach to develop functional replacements for tissues such as articular cartilage, auricular cartilage, bone, intervertebral disc, tendon, and ligament. Our lab has developed various technologies to enable tissue engineering research, such as microencapsulation, photochemical crosslinking, biomimetic and functional materials, and micropipette-based mechanical loading.
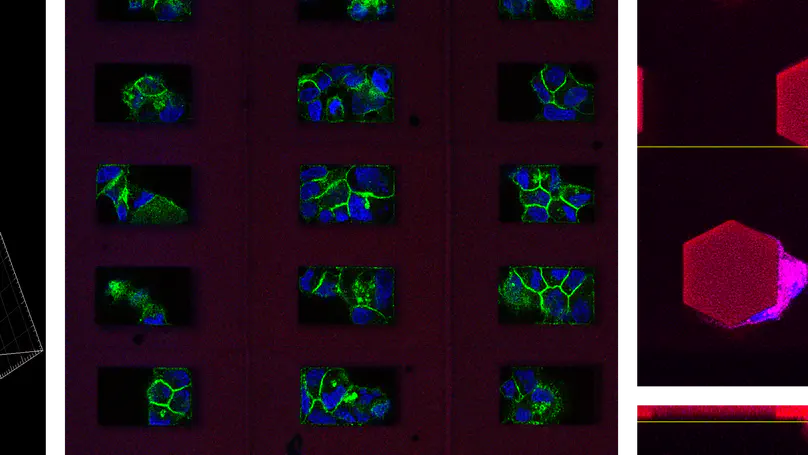
Cells are living entities that sense and respond to many signals present in their microenvironment. We are developing the Multiphoton Microfabrication and Micropatterning (MMM) platform to engineer and precisely control each facet of the cell niche, including mechanical properties such as stiffness and elastic modulus, the 3D spatial position and concentration of extracellular matrix (ECM), growth factor and cell-cell interaction proteins, and high-resolution topographies. This enables us to pinpoint and research the effect of individual and combined factors in the microenvironment on cell behavior, for example, in the cell preference for ECM selection, phenotype maintenance, asymmetric cell division, and others.
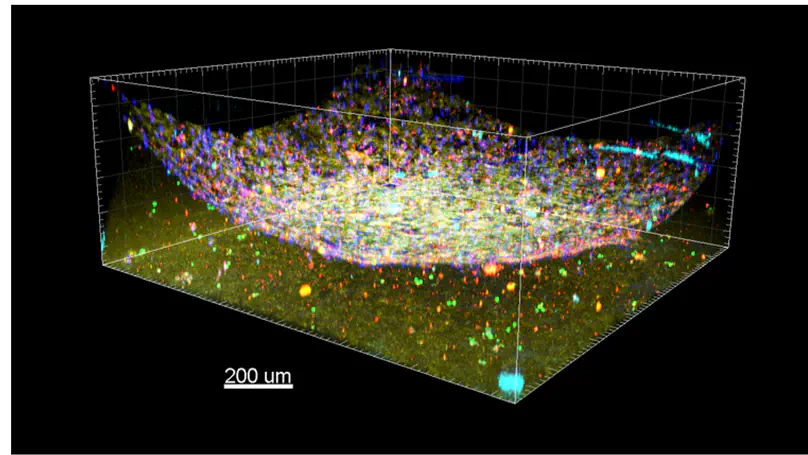
Cells behave differently when they are cultured in 3D configuration, which is particularly important when evaluating their response to drugs and interventions. We are developing different 3D organoid models for different cell types to fully reflect their physiological response to specific drugs. These models can be used for improving the effectiveness of therapies in personalized medicine.

We are interested in studying the mechanosensing and signalling mechanisms of stem cells in physiologically relevant matrices. Particularly, we study cell-matrix interactions, cell-cell interactions, and cytoskeletal responses of cells under active mechanical loading and in microenvironments with tunable mechanical stiffnesses. For example, using bioreactor technologies with different loading modes, we can study the behavior of stem cells encapsulated in collagen microspheres.
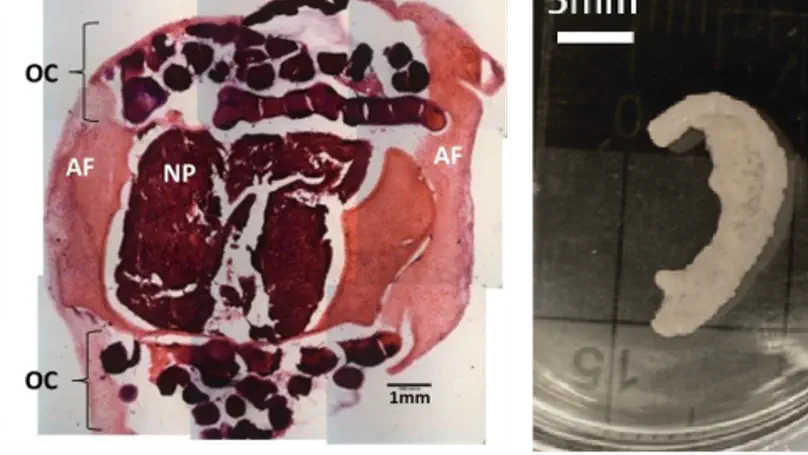
Biomimetic scaffolds mimic important features of the extracellular matrix (ECM) and can be finely controlled at the nano- or microscale. Rational design of biomimetic scaffolds is based on consideration of the ECM as a natural scaffold; ECM provides a niche for cell adhesion, migration, proliferation, and differentiation. We are working on fabrication of different biomimetic scaffolds that recapitulating the composition, structure, and function of the native tissues. For example, we are developing a collagen microspheres-based scaffold for mimicking the ear cartilage; and a glycosaminoglycans (GAGs)-rich scaffold to mimic the ECM of the nucleus nucleus pulposus and cartilage. These biomimetic scaffolds can be used for tissue regeneration.

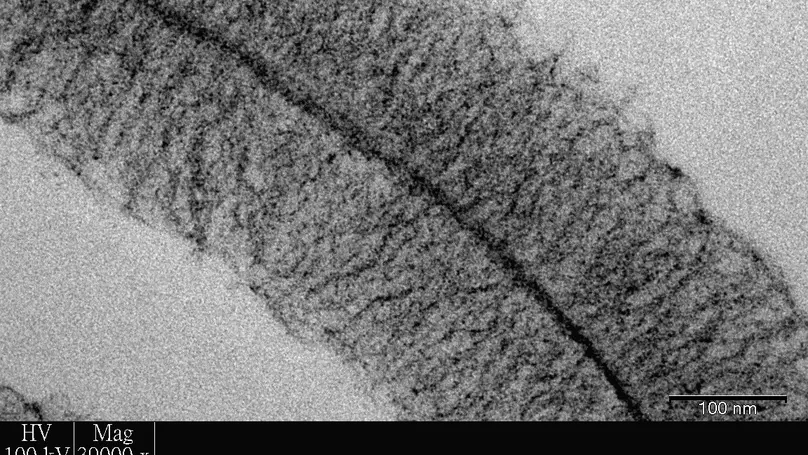
Microencapsulation is the process to entrap cells within the confines of a semi-permeable membrane or a homogenous solid matrix. We have developed a microencapsulation technology to entrap cells within a dense meshwork of extracellular matrix (ECM) components, such as nano-sized collagen fibers, laminin, fibronectin, and glycosaminoglycans (GAGs). This meshwork is able to support growth and proliferation, migration, and differentiation of cells. Many cell types including mesenchymal stem cells, embryonic stem cells, chondrocytes and fibroblasts have been successfully encapsulated. The microspheres are being developed for tissue engineering applications. We are also developing biomimetic and functional materials for use in various tissue engineering applications.
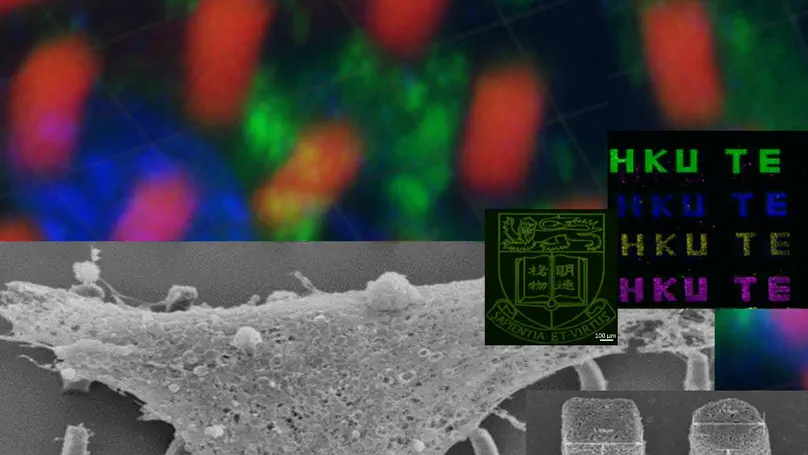
Multiphoton Microfabrication and Micropatterning (MMM) is a platform technology that utilizes a femto-second infrared laser to create 3D micro-structures and micropatterns with sub-micron resolution, in a precisely, quantitatively and spatially controllable manner. The laser photoactivates a photosensitizer and crosslinks the materials of interest, solidifying the materials in a liquid bath. Multiphoton excitation is confined to the tiny focus of the laser beam, achieving sub-micron resolution in the micro-fabricated structures. Owing to the low phototoxicity of the multiphoton laser and the non-thermal nature of photochemical crosslinking, a wide range of biomedical applications can be developed. For example, we can microfabricate delicate biomaterials such as proteins and microstructures within and around live cells.

We have developed a series of micropipette and microplate-based techniques to measure the mechanical properties of individual cells and tissue micro-masses and to investigate the cellular responses to mechanical signals. These techniques facilitate mechanical characterization of cells and small tissue micro-masses and assist rational design of bioreactors in developing functional tissue replacements.
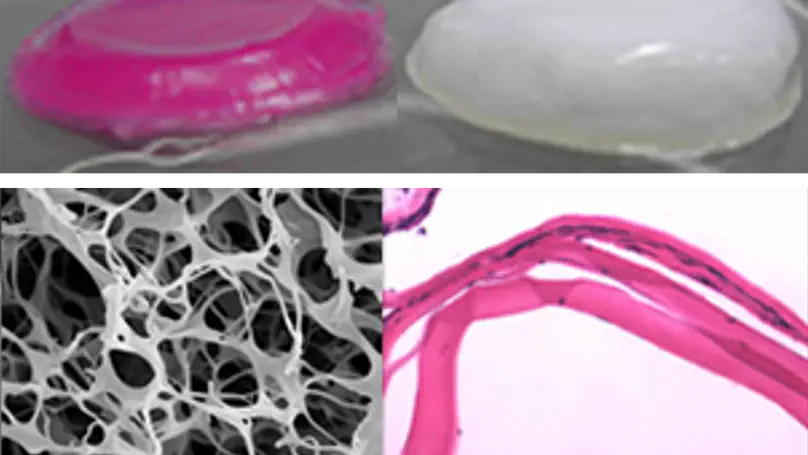
Photochemical crosslinking is a processing technique for collagen-based biomaterials involving photochemical reactions of light-activated photosensitizers. This technology is able to modify the physicochemical properties of collagen-based biomaterials including mechanical stability and swelling characteristics without compromising their biocompatibility. This broadens the applications of collagen-based biomaterials in tissue engineering.