Mechanotransduction and vascular function
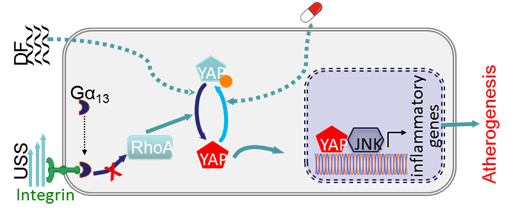
Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow
The Yorkie homologues YAP and TAZ, effectors of the Hippo pathway, have been identified as mediators for mechanical stimuli. We have recently demonstrated that endothelial YAP/TAZ activity is regulated by different patterns of blood flow, and YAP/TAZ inhibition suppresses inflammation and retards atherogenesis. Atheroprone-disturbed flow increases whereas atheroprotective unidirectional shear stress inhibits YAP/TAZ activity. Unidirectional shear stress activates integrin and promotes integrin-Gα13 interaction, leading to RhoA inhibition and YAP phosphorylation and suppression. YAP/TAZ inhibition suppresses JNK signaling and downregulates pro-inflammatory genes expression, thereby reducing monocyte attachment and infiltration. In vivo endothelial-specific YAP overexpression exacerbates, while CRISPR/Cas9-mediated Yap knockdown in endothelium retards, plaque formation in ApoE-/- mice. We also show several existing anti-atherosclerotic agents such as statins inhibit YAP/TAZ transactivation. Our results indicate that integrin-Gα13-RhoA-YAP pathway holds promise as a novel drug target against atherosclerosis. This novel finding has just been published in Nature (2016 Dec 7. doi: 10.1038/nature20602. [Epub ahead of print]) and commented in Nature News and Views (2016 Dec 7. doi: 10.1038/nature20489. [Epub ahead of print]).
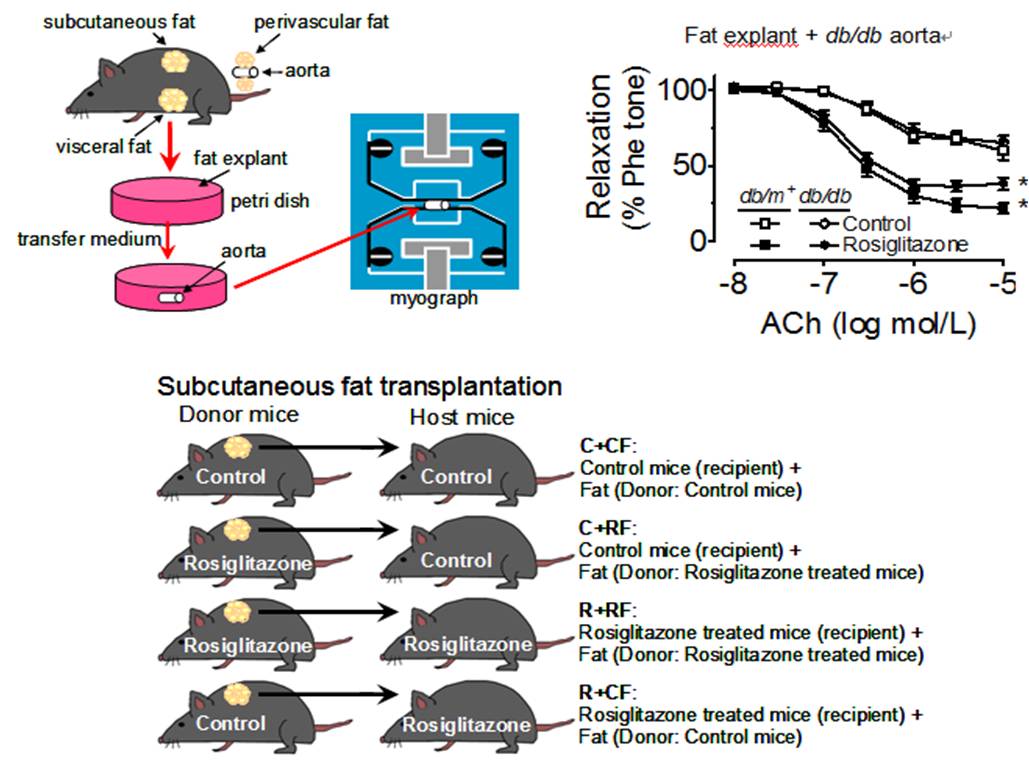
Adipo-Vascular Axis ¡V Adiponectin Restores Endothelial Function in Diabetes
Adipose tissue is increasingly recognized as an important metabolic and endocrine organ in the regulation of glucose metabolism. Dysregulation of adipose tissue participates in the development of insulin resistance and vascular complications of diabetes. PPAR£^ ligands improve insulin sensitivity in diabetes. This study with focus on the ¡§adipose-vascular loop¡¨ demonstrates that adipose tissue-derived adiponectin plays an obligatory role in the PPAR£^ agonists-induced improvement of endothelial function in diabetic and diet-induced obese mice. Adiponectin increases NO bioavailability by activating AMPK and cyclic AMP/PKA signaling. Our results support a differential role of various fat depots, which is directly related to the amount of adiponectin released upon PPAR£^ activation. Adipose tissue could be an important intervention target for newly developed PPAR£^ agonists in the alleviation of diabetic vasculopathy. The innovative experimental approaches employed in our investigation help open up an emerging research area on adipokines-vascular regulation as effective therapeutic targets for new anti-diabetic and anti-hypertensive drugs [Wong et al., Cell Metabolism 2011;14(1):104-115, http://www.ncbi.nlm.nih.gov/pubmed/21723508].
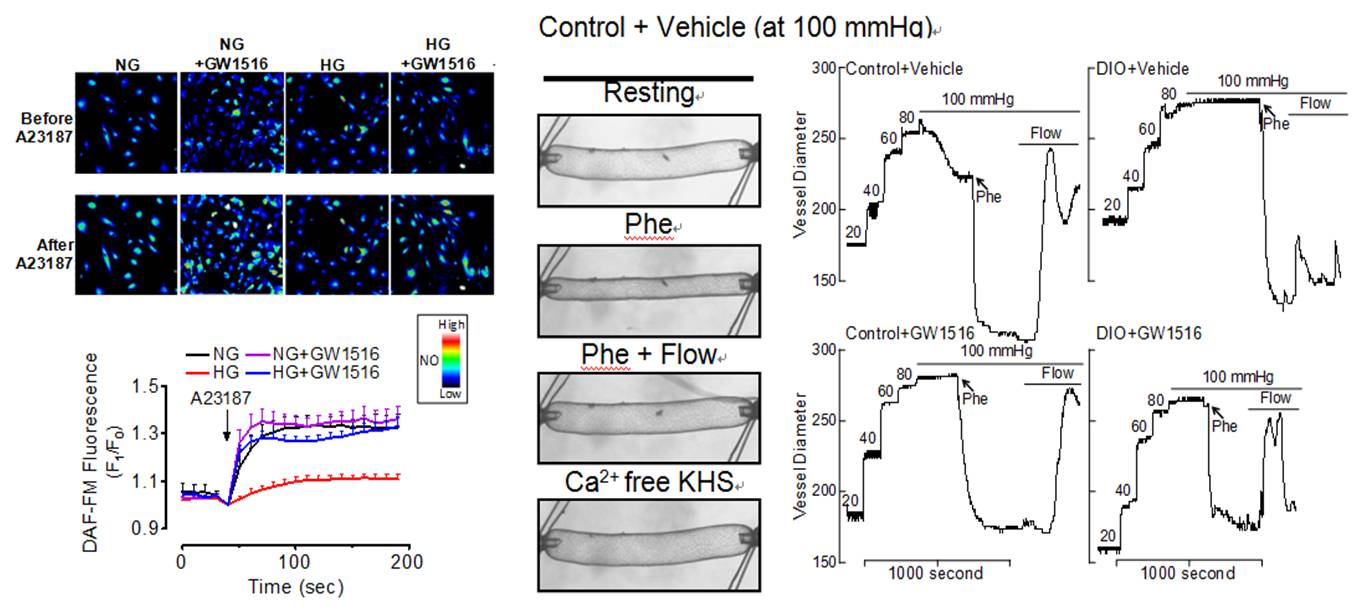
PPAR Research
Recent evidence highlights the therapeutic potential of peroxisome proliferator-activated receptor-£_ (PPAR£_) agonists to increase insulin sensitivity in diabetes. However, the role of PPAR£_ in regulating vascular function is incompletely characterized. Treatment with PPAR£_ agonist GW1516 restores the impaired endothelium-dependent relaxations in mouse aortae under high-glucose conditions or in db/db mouse aortae ex vivo. Oral treatment with GW1516 improves the endothelium-dependent relaxations in aortae and flow-mediated vasodilatation in mesenteric resistance arteries of obese mice in a PPARd-specific manner. The effects of GW1516 on endothelial function were mediated through PI3K and Akt with a subsequent increase of eNOS activity and NO generation. Our study demonstrates an endothelial-protective effect of PPAR£_ agonists in diabetic mice through PI3K/Akt/eNOS signaling cascade, suggesting therapeutic values of PPAR£_ agonists for diabetic vasculopathy [Tian et al., Diabetes 2012;61(12):3285-3293, http://www.ncbi.nlm.nih.gov/pubmed/22933110].
An elevated plasma level of 5-HT or up-regulation of 5-HT receptor signaling or both are implicated in vascular contraction and remodeling in pulmonary arterial hypertension. Our recent study demonstrates the beneficial effect of PPAR£^ activation on 5-HT 2B receptor-mediated vasoconstriction, providing a new mechanism for the potential use of PPAR£^ agonists in treating pulmonary arterial hypertension [Liu et al., Hypertension 2012;60(6):1471-1478, http://www.ncbi.nlm.nih.gov/pubmed/23108648]. PPAR£^ agonist up-regulates the expression of endothelin B receptor [ET(B)R] in mouse aortas and human vascular endothelial cells through enhancing ET(B)R gene promoter activity via directly binding to ET(B)R gene promoter. In vivo treatment with rosiglitazone attenuates the endothelin-1-induced constrictions and increases the ET(B)R expression in mouse arteries, suggesting the potential therapeutic value of PPAR£^ agonists. [Tian et al., Hypertension 2010;56(1):129-135, http://www.ncbi.nlm.nih.gov/pubmed/20516393]. Indeed, the PPAR£^-dependent increase in NO bioavailability accounts for the angiotensin type 1 receptor-independent vascular benefits of telmisartan [Yuen et al., Cardiovascular Research 2011;90(1):122-129, http://www.ncbi.nlm.nih.gov/pubmed/21156825].
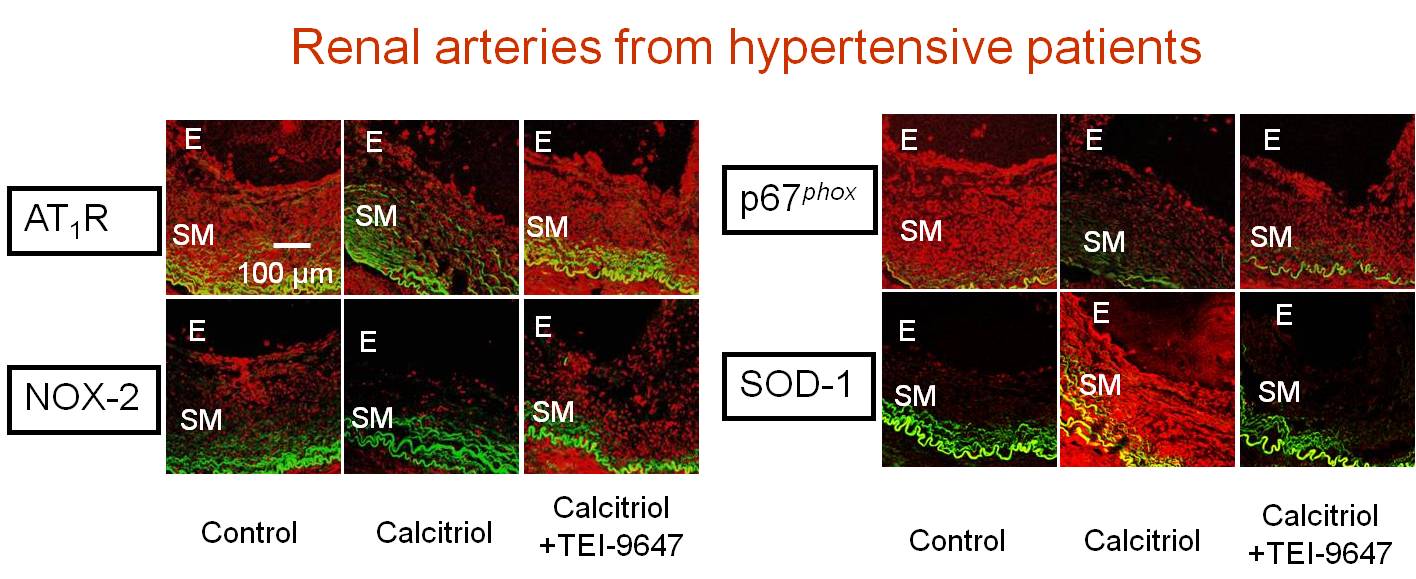
Vascular Benefit of Vitamin D
An inverse correlation exists between the vitamin D level in the blood and the incidence of heart failure, cardiovascular mortality, and elevation of arterial blood pressure. We provide the first line of evidence showing that chronic treatment with calcitriol, an active form of vitamin D, protects against renovascular function in hypertension. The calcitriol-induced protection is likely to be mediated by activation of the vitamin D receptor leading to the down-regulation of the expressions of angiotensin type 1 receptors, NAD(P)H oxidase subunits and up-regulation of antioxidant enzymes SOD-1 and SOD-2. These in turn prevent the ROS overproduction. The findings in human renal arteries and human endothelial cells are confirmed both by in vitro and in vivo results in hypertensive rats [Dong et al., European Heart Journal 2012;33(23):2980-2990, http://www.ncbi.nlm.nih.gov/pubmed/22267242]. Cardiovascular risks increase in the postmenopausal women and vitamin D is supplemented for osteoporosis. Calcitriol restores endothelial function and renal blood flow through normalizing the over-expression of COX-2 and thromboxane-prostanoid receptors in renal arteries during estrogen deficiency in ovariectomized rats. [Dong et al., Kidney International 2013; 84(1):54-63 with Commentary 2013;84(1):9-11, http://www.ncbi.nlm.nih.gov/pubmed/23423254]. Our studies suggest calcitriol and vitamin D receptor activation as a novel therapeutic strategy to ameliorate vascular dysfunction associated with hypertension and estrogen deficiency.
From Skeleton to Cytoskeleton: Osteocalcin Transforms Vascular Fibroblasts to Myofibroblasts
In contrast to the current view that vascular dysfunction progresses from ¡§inside-out,¡¨ recent evidence suggests an ¡§outside-in¡¨ hypothesis, proposing that inflammation can originate from the adventitia and develop toward the intima, resulting in vascular wall thickening. Vascular remodeling events such as neointima formation are characterized by the acquisition of migratory and proliferative ability of fibroblasts after their transformation into myofibroblasts. Toll-like receptor 4 expression has been reported in human and murine arterial lesions and intimal hyperplasia. Although cumulating evidence implies a cross-talk between bone pathology and cardiovascular diseases, there is a lack of clear mechanism on how bone-associated proteins such as osteocalcin affect the vasculature. Our recent study elucidate that in human neointimal lesions, the pro-inflammatory markers TLR4 and COX-2 are co-expressed along with the proteins involved in remodeling such as a-smooth muscle actin and fibronectin. This study also identifies a clear mechanistic pathway in which osteocalcin triggers adventitial fibroblast to produce and release angiotensin II. This in turn activates PKCd, TLR4, and COX-2 to transform fibroblasts into myofibroblasts, thus, supporting a pathogenic linkage between the skeletal hormone and vascular remodeling [Yuen et al., Circulation Research 2012;111(3)e55-e66, http://www.ncbi.nlm.nih.gov/pubmed/22679141].
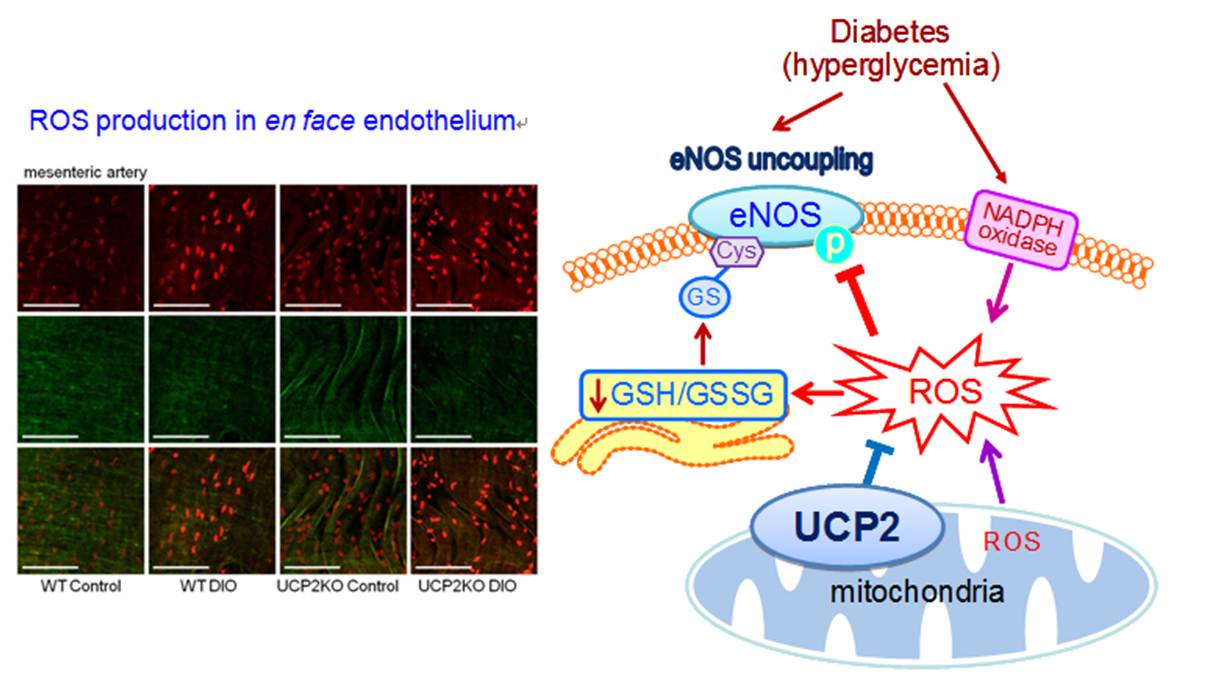
UCP2, Oxidative Stress, and Vascular Regulation
The uncoupling protein-2 (UCP2) is localized to the inner membrane of the mitochondria and has been found to reduce mitochondrial ROS production, thus acting as an antioxidant protein. Our recent study demonstrates that UCP2 over-expression augments both endothelium-dependent relaxations in conduit artery and flow-mediated dilatation in resistance artery in obese diabetic mice. This vascular benefit is associated with the increased NO bioavailability, whereas UCP2 deficiency exaggerates oxidative stress and endothelial dysfunction in obese mice. Our new findings highlight a protective role of UCP2 against endothelial dysfunction in diabetes and obesity. [Tian et al., Circulation Research 2012;110(9):1211-1216, http://www.ncbi.nlm.nih.gov/pubmed/22461387]. We also confirm the clinical efficacy of renin-angiotensin system inhibitors in diabetic mice through curtailing angiotensin type 1 receptor-associated oxidative stress in conduit and resistance vessels [Wong et al., Antioxidants & Redox Signaling 2010;13(6):757-768, http://www.ncbi.nlm.nih.gov/pubmed/20136508].
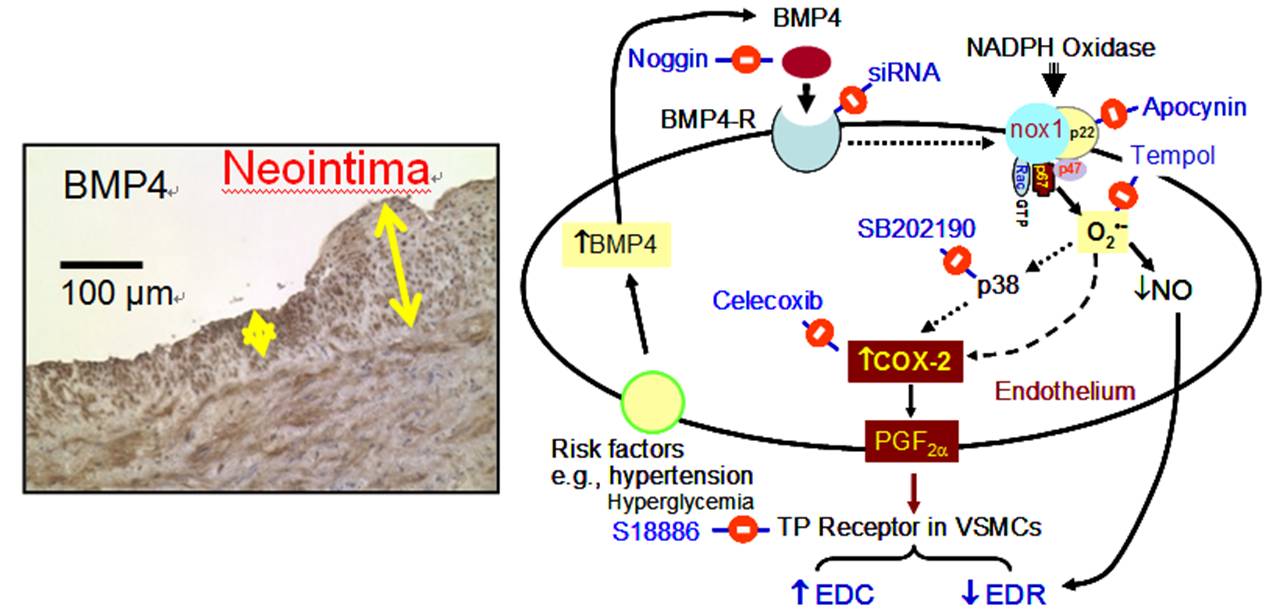
Bone-Vessel Crosstalk - BMP4 Triggers Endothelial Dysfunction
Bone morphogenic protein-4 (BMP4) is a pro-inflammatory gene induced by a disturbed flow in endothelial cells. We demonstrate for the first time that BMP4 exaggerates endothelium-dependent contractions in mouse aortas whereas the silencing of BMP receptor 1A prevents the harmful effects of BMP4. BMP4 elevates ROS in mouse arteries and endothelial cells, which in turn activates p38 MAPK and contributes to BMP4-induced COX-2 up-regulation and altered vascular reactivity. BMP4-induced endothelial dysfunction is absent in COX-2-deficient mice. We verify positive association of BMP4 and COX-2 in endothelial dysfunction in hypertensive rats and patients. BMP4 and its downstream oxidative stress-dependent up-regulation of COX-2 expression and activity are important in the development of hypertension. Strategies specifically targeting BMP4-COX-2 signaling cascade are potentially useful in combating vascular dysfunction related to human hypertension [Wong et al., Circulation Research 2010;107(8):984-991, http://www.ncbi.nlm.nih.gov/pubmed/20724703]. We also provide novel findings supporting the pathological role of COX-2 and BMP4 in vascular dysfunction in rat model of renovascular hypertension [Tian et al., Antioxidants & Redox Signaling 2012;16(4):363-373, http://www.ncbi.nlm.nih.gov/pubmed/21951274]. In addition, we delineate that BMP4 causes endothelial cell apoptosis through activation of caspase-3 in a ROS/p38MAPK/JNK-dependent signaling cascade [Tian et al., Journal of Molecular and Cellular Cardiology 2012;52(1):237-244, http://www.ncbi.nlm.nih.gov/pubmed/22064324].
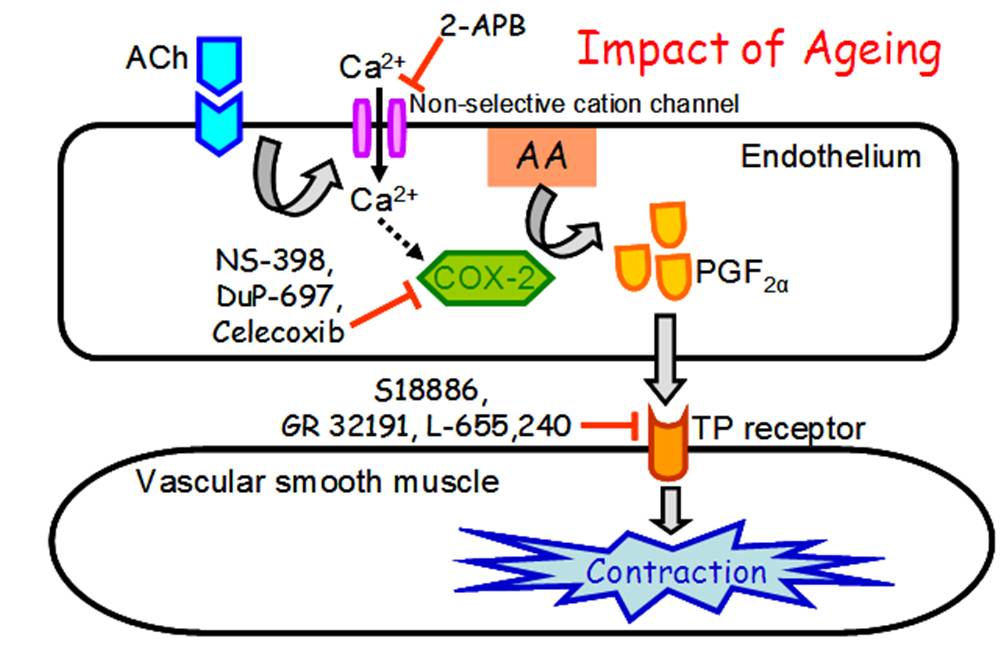
PGF2£\ as the Endothelium-derived Contracting Factor
Hypertension and vascular dysfunction result in the increased release of endothelium-derived contracting factors (EDCF), the identity of which is poorly defined. This study was the first to clearly demonstrate that COX-2-mediated PGF2£\ acts as a novel EDCF in blood vessels. Our results reveal a crucial role of COX-2 in endothelium-dependent contractions with an increased importance during ageing and, possibly, that it has a similar relevance in humans. The significance of our novel findings was commented on by the editorial of Circulation Research as follows. ¡§First, it should be noted that COX-2 was the main enzyme involved in EDCF, i.e., PGF2£\ release in the hamster aorta. In humans, COX-2 rather than COX-1 produces the majority of vascular prostaglandins in healthy and atherosclerotic areas. Thus, the prostanoid synthetic pathways in humans appear to be closer to the hamster than the rat, as also suggested by the authors. Overall, Wong et al have demonstrated convincing evidence for COX-2-derived PGF2£\ as a new EDCF acting via TP receptor in the hamster aorta. This provides pharmacological evidence for TP receptor antagonists as useful tools to correct imbalances between EDRF/EDCF. This could be an argument for the revival of the concept of thromboxane receptor blockade to prevent regional ischemia.¡¨ [Wong et al.,Circulation Research 2009;104(2):228-235, http://www.ncbi.nlm.nih.gov/pubmed/19096033]. Besides, we have highlighted a critical role of PKC£_ in mediating angiotensin II-induced COX-2 over-expression, which promotes the release of vasoconstrictors and a pro-atherosclerotic cytokine, monocyte chemoattractant protein-1. Because PKC£_ is preferentially activated in diabetes, our findings suggest that PKC£_ may be a promising target for therapeutic intervention in COX-2-mediated vascular complications. [Wong et al., Arteriosclerosis, Thrombosis and Vascular Biology 2011;31(5):1169-1176, http://www.ncbi.nlm.nih.gov/pubmed/21311042].