Research Interest - Click Chemistry
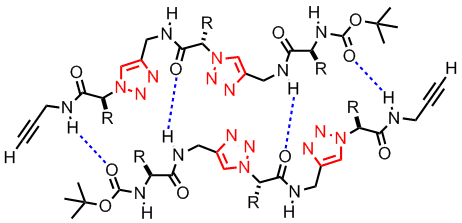
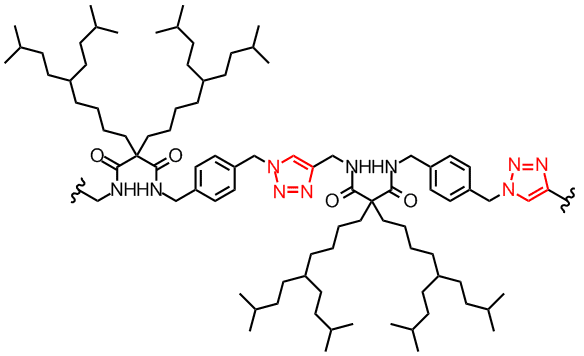
(L to R): Head-to-tail dimeric structure of a click tetrapeptide, a click dendronized polymer
Click Chemistry
Click Chemistry 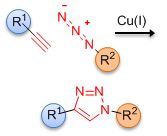
Click chemistry represents a special type of chemical reactions that can join two separate molecular species together in a highly efficient manner, and without the formation of any by-products. There are many reactions that can be considered as click chemistry, among them the Cu(I) catalyzed cycloaddition reaction between a terminal acetylene and an azide to form a 1,4-disubstituted-1,2,3-triazole is of particular interest. This chemistry was developed independently by Meldal[1] and Sharpless,[2] and has found numerous applications in medicinal and materials chemistry.
The triazole ring has been proposed to bear some structural similarities to the amide functionality. Furthermore, the lone pairs on the nitrogen atoms and the acidic triazole C-H bond constitute the typical characteristics of a H-bonding unit. As a result, we are particularly interested in examining the supramolecular chemistry of compounds containing multiple triazole units.[3]
Click Dendronized Polymers 
Many interesting properties of polymers, such as their liquid crystalline, non-linear optical, and optoelectronic properties relied heavily on their self assembling behavior in the bulk state. Understanding the fundamental factors that govern their self assembling process is very important for the design of polymers for specific applications. Our key objectives are to identify new factors that control the polymer self assembly process, and to design new polymer molecules with pre-determined self assembling properties. One area that we have been working extensively concerns with dendronized polymers containing a poly(amide-triazole) backbone. We would like to investigate the effect of the amide and triazole units on the polymer self assembly and gelating properties. These dendronized polymers could be conveniently prepared via click polymerization.[4] Our investigations revealed that a synergistic self association effect due to the presence of many amides and triazole units in close proximity along the polymer chain. Further studies showed that the self assembly and gelation strength of these polymers was controlled by the size of the dendron side chains, the relative arrangement (centrosymmetric vs non-centrosymmetric) and the number of the amide and triazole units along the polymer backbone.
Click Peptidomimetics 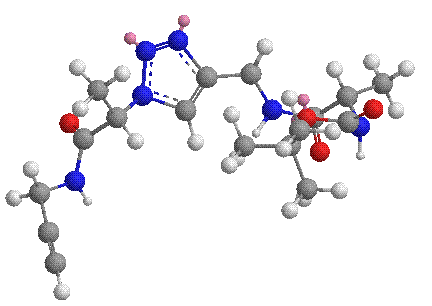
A second area that we have been working is click peptidomimetics. These are compounds prepared by the click coupling of alkyne functionalized a-amino acid derivatives and a-azido acid compounds. The novelty of this approach is that the click coupling reaction is highly specific and therefore no protective group chemistry is needed for the amino acid side chains. Furthermore, it was envisaged that these compounds should exhibit very interesting and rich supramolecular chemistry due to the H-bonding and dipole-dipole interactions of the triazole and amide moieties. For example, we have shown that such compounds formed head-to-tail dimers in organic solvents.[5] We are also investigating the possibility of building novel conformations such as b-turn, helical structure using click chemistry.
References:
[1] J. Org. Chem. 2002, 67, 3057.
[2] Angew. Chem. Int. Ed. 2002, 41, 2596.
[3] ChemCommun. 2010, 3437.
[4] (a) Angew. Chem. Int. Ed. 2008, 48, 6912; (b) Chem. Eur. J. 2011, 17, 8395.
[5] Org. Lett. 2012, 14, 394.
Focused Projects
