Research Interest - Nonplanar Nanographenes
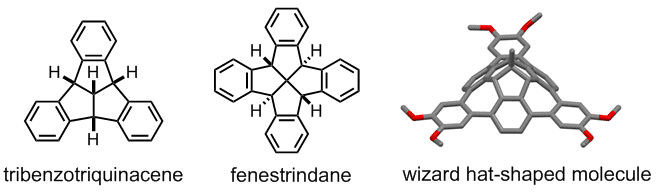
(L to R): Structure of tribenzotriquinacene (TBTQ), fenestrindane and a wizard hat shaped molecule derived from TBTQ
Nonplanar Nanographenes
Nonplanar Nanographenes 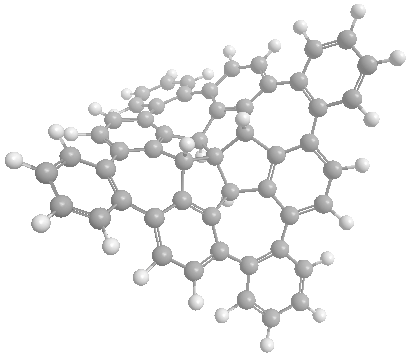
Polycyclic aromatic compounds (PACs) possessing a non-planar p-surface have aroused considerable interests due to their strained structures and unusual properties[1]. Curved PACs in the forms of spheres, tubes, hemi-spheres and saddles are well-known morphologies of nanographenes and their applications have been well-documented[2].
We are interested in investigating the supramolecular properties such as self-assembling, stacking and host-guest binding interaction of these non-planar nanographenes. Furthermore, due to their highly extended conjugation structure, it is expected that they also show interesting optoelectronic properties.
So the question is how to induce non-planarity in a conjugated molecule? Traditionally this can be achieved by two methods. The first is the introduction of non-hexagonal rings to a graphene molecule, while the second is to induce steric contortion via steric crowding among the peripherial groups of a graphene unit.
Our method of creating curved nanographenes is to introduce sp3-hybridized carbon atoms in a nanographene skeleton. The structural units that we chose to accomplish this are tribenzotriquinacene (TBTQ) and fenestrindane. They both have a rigid non-planar structure that serve to twist the graphene plane. So the next question is how to achieve this synthetically?
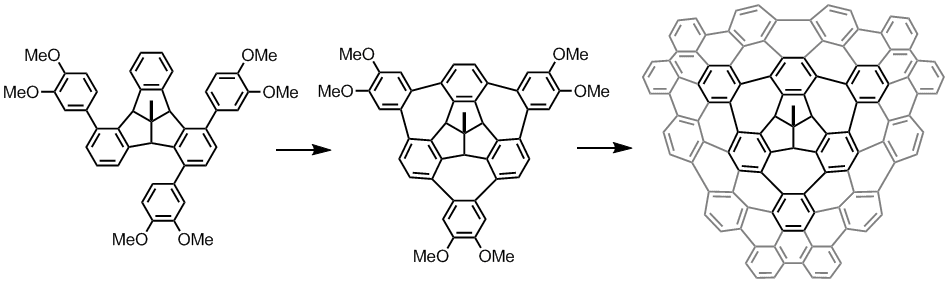
Tribenzotriquinacene Derivatives 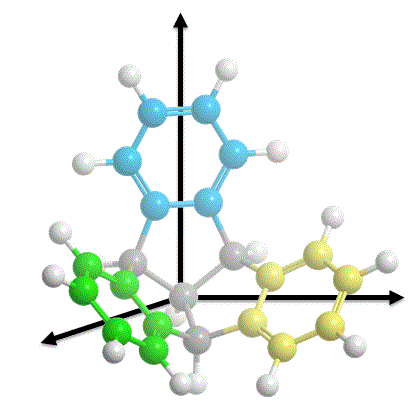
TBTQ is a structural interesting molecule which contains three isolated aromatic rings that are posed nearly orthogonal to each other in the three dimensional space. We need to develop new synthetic methods that (a) can produce TBTQ compounds with different kinds of functionalities [3], and (b) can connect these three aromatic rings via conjugated elements such as additional aromatic rings to form a conjugated polyaromatic system. We recently reported that by using a Scholl oxidative cyclization method, a wizard hat-shaped molecule could be synthesized. Furthermore, the three isolated aromatic rings in the original TBTQ skeleton now become conjugated via three additional aromatic rings [4]. The new p-extended molecule can serve as a building block towards the synthesis of much larger non-planar nanographene systems.
Fenestrindane Derivatives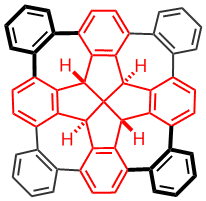
Fenestrindane is another structural interesting molecule (shown in red in the left structure). It possesses four indane wings that are disposed in a non-coplanar fashion. In particular, the east and west aryl rings, and the north and south aryl rings are orthogonal to each other. We are trying to develop new synthetic routes to p-extended fenestrindane compounds [5] that possess a saddle shape, and to examine the self-assembly and optoelectronic properties of such warped nanographenes.
References:
[1] (a) Angew. Chem. Int. Ed. 1992, 31, 766; (b) Chem. Commun. 2012, 48, 9882; (c) Chem. Soc. Rev. 2015 , 44, 6464.
[2] (a) Chem. Rev. 2015 , 115, 7046; (b) Chem. Soc. Rev. 2015 , 44, 6616; (c) Angew. Chem. Int. Ed. 2016 , 55, 5136.
[3] (a) J. Org. Chem. 2013, 78, 1062; (b) J. Org. Chem. 2014, 79, 9335; (c) Chem. Asian J. 2015, 10, 1150; (d) Chem. Eur. J. 2015, 21, 12011; (e) J. Org. Chem. 2016, 81, 2308; (f) J. Org. Chem. 2017, 82, accepted. DOI: 10.1021/acs.joc.6b02326.
[4] (a) J. Am. Chem. Soc. 2016, 138, 13778; (b) Org. Chem. Front. 2017, 4, 817.
[5] (a) Synlett 2016, 27, 1255; (b) Angew. Chem. Int. Ed. 2017, 56, 12356.
Focused Projects
