Research Interest - Molecular Self Assembly
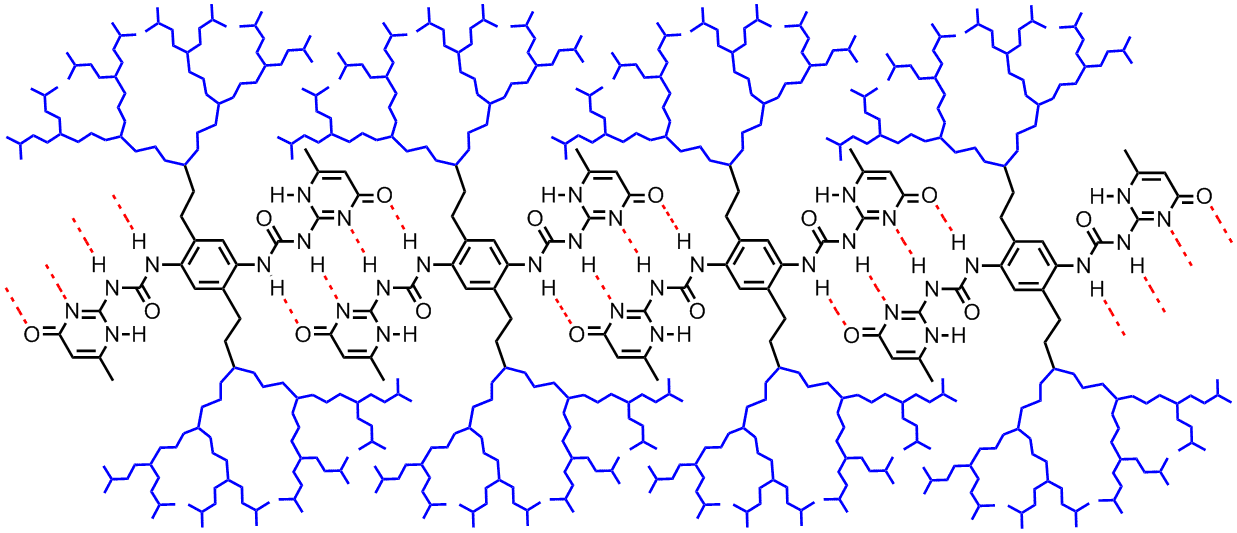
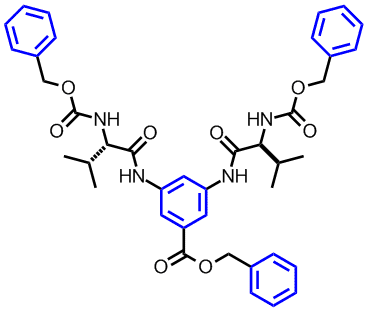
(L to R): Structure of a H-bond mediated supramolecular dendronized polymer, a low molecular weight organogelator
Molecular Self Assembly
Molecular Self Assembly 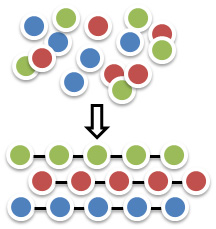
Self assembly is a thermodynamic-driven process in which a group of randomly oriented molecules organize themselves into a specific pattern or an order structure in the absence of external directive forces. These molecules are normally inscribed with functionalities that can promote internal, weak but specific interactions (e.g. H-bonding, hydrophobic, p-p interactions) among themselve to form organized hierarchical architecture. Molecular self assembly can be found everywhere in nature. The formation of crystals, micelles, lipid-bilayers, DNA double helices are all self assembly processes. We are particularly interested in the design of self assembling molecular systems that can self organize into well defined materials and in the exploration of new frontiers and principles in supramolecular chemistry.
Gelation 
Gels are jelly-like substances which are wet and soft. They appear like a solid, and also behave as a very viscous liquid. Gelation of a solvent by a gelator occurs through self-assembly of the gelator molecules into elongated fiber like structures, which then forms an entangled three-dimensional network in the solvent. As a result, these networks immobilize the solvent through capillary forces within the pores. Gels can be divided into chemical and physical gels. In a chemical gel, the three-dimensional gel network is formed via covalent bonds and gelation is therefore an irreversible process. In contrast, the network of a physical gel is built up from small molecular subunits, which are held by non-covalent interactions and is thus a reversible process.
Our focus the gelation research can be divided into two areas. The first is concerned with the preparation of low molecular weight organogelators based on H-bonding and p-p interactions.[1-3] We have demonstrated that aromatic pendant groups[2] and structural rigid functionalities[3] could enhance the organogelating properties of amino acid based organogelators in aromatic solvents. The second area is related to the preparation of polymer physical gels and elucidation of their gelation mechanism. For example, we found out that the gelation strength of polymer physical gels depends on the density of the interacting groups, the relative orientation of the dipoles of the polymer repeating unit and the size of the side chain appendages.[4]
Self Assembling Dendrimers and Dendronized Polymers 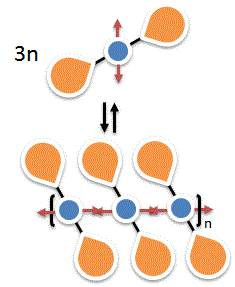
The use of non-covalent interactions to build higher order, structurally defined and system reversible self assembling dendrimers and dendronized polymers is one of our major research focus areas. We demonstrated that during the construction of such supramacromolecular species, the size and microenvironmental effects of the dendritic appendages could play a very influential role in dictating the outcome of the self assembling process, and one cannot simply consider such innocent appendages as inert functionalities.[5] We are currently exploring other means, in addition to H-bond interactions, to build up such self assembling systems.
References:
[1] (a) Chem. Eur. J. 2005, 11, 5817; (b) Tetrahedron 2005, 61, 11279; (c) Beilstein J. Org. Chem. 2010, 5, 1015.
[2] Tetrahedron 2007, 63, 363.
[3] Tetrahedron 2007, 63, 7407.
[4] (a) Angew. Chem. Int. Ed. 2008, 47, 6912; (b) Chem. Eur. J. 2011, 17, 8395; (c) Chem. Eur. J. 2013, 19, 2478; (d) Chem. Eur. J. 2013, 19, 15735; (e) Chem. Commun. 2014, 50, 3064.
[5] (a) Org. Lett. 2006, 8, 1811; (b) Chem. Asian J. 2010, 5, 2249; (c) Macromolecules 2010, 43, 8389; (c) Chem. Commun. 2015, 51, 2349.
Focused Projects
