Bone Laboratory

Fracture healing
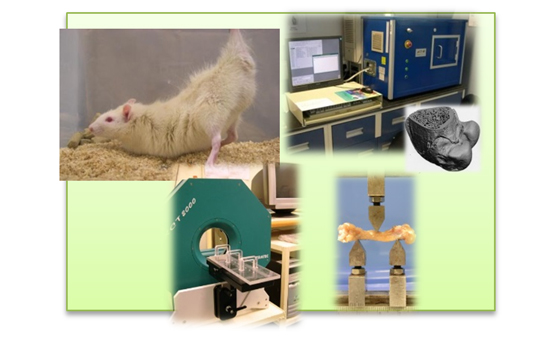
A herbal paste containing Carthami Flos, Dipsaci Radix, Notoginseng Rhizoma and Rhei Rhizoma (CDNR) has been developed to treat fracture topically. It was nanomized (collaborated with UST) or further simplified to a 3-herb formula (CDR). Fracture and drill-defect animal models in rabbit and rat, respectively, have been developed to study their efficacy. The in vivo efficacy of these formulae were assessed using various of methods including X-ray, micro-CT, biomechanical test and histomorphometry. CDR paste has been translated into patch form for a clinical study with the Department of Orthopaedics and Traumatology (ORT) under the support of Innovation and Technology Fund (ITF). It decreased the fracture length at the fifth metatarsal fracture and ameliorated the pain of patients.

Soft tissue injury
Paw edema, muscle contusion and rotator cuff tear rat models have been established in our Institute to study the soft tissue injury. A topical herbal bath formula containing Carthami Flos, Angelicae Sinensis Radix and Achyranthis Bidentatae Radix (CAA), has been developed and proven effective for the swelling control of paw edema. The nanomized CDNR and CDR pastes were also found effective to promote the recovery of the soft tissue injury. Assessments including water displacement test, allodynia threshold, histology and immunohistochemistry were employed. Zebrafish embryo model was also recruited to study their proangiogenic effect. In vitro platforms including anti-inflammation study on RAW264.7, angiogenesis study on HLEC and HUVEC (cell migration and tube formation) were also utilized. A clinical study collaborated with ORT under the ITF support showed that CDR patch and CAA bath improved the pain score and foot function index of patients suffering from plantar fasciitis and acute ankle sprain injury, respectively. CAA also reduced the edema thickness of the patients concurrently.
Osteoporosis
A herbal formula formed with Epimedii Herba, Ligustri Lucidi Fructus and Psoraleae Fructus (ELP) was designed for the prevention of osteoporosis. The efficacy of this formula was studied comprehensively through in vitro studies (including seropharmcological approach), in vivo studies (OVX and tail-suspension rat models have been developed. Assessment platforms including pQCT, micro-CT, analysis of biochemical markers in serum and biomechanical test) and clinical trial. Both of the in vivo studies and clinical trial demonstrated that ELP was effective to reduce bone loss during the development of osteoporosis. The in vitro studies illustrated that ELP prevented bone loss through stimulating osteogenesis and reducing osteoclastogenesis concurrently.